Abstract

Introduction. The clinical benefits of lenalidomide in patients with CLL are well established. Lenalidomide inhibits CLL-cell proliferation, enhances T-cell synapse formation and CD154 expression, and modulates the leukemia cell microenvironment. Although clinically beneficial, side effects such as infection due to myelosuppression, diarrhea, peripheral neuropathy, and skin rash occasionally impose treatment discontinuation. Because lenalidomide reduces PD-1 and PDL-1 expression and induces CLL-T-cell synapse formation, we sought to determine whether, similar to checkpoint inhibitors, the clinical effect of lenalidomide persists after treatment discontinuation.
Methods. We analyzed the clinical characteristics of patients with CLL who were enrolled in clinical trials with lenalidomide at the MD Anderson Cancer Center (MDACC) and discontinued lenalidomide because of toxicity. In these frontline or salvage trials, lenalidomide was administered as a single agent or in combination with anti-CD20 monoclonal antibodies. Time to next treatment (TTNT) was calculated from the date of lenalidomide discontinuation to the date of subsequent treatment or last follow-up, and a univariate analysis and a log-rank test was performed.
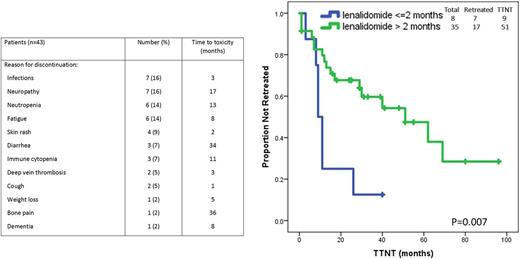
Results. Lenalidomide was discontinued due to toxicity in 43 of 208 patients treated. The median age of those patients was 66 (range, 42-83); 22 (52%) patients were men; 40% had advanced Rai stage, 52% had β2M of ≥4 mg/L; 57% had unmutated IGHV, and 21% had unfavorable cytogenetic abnormalities [including del(11q) or del(17p)]. Fourteen patients (31%) received single agent lenalidomide and 29 patients (69%) received lenalidomide in combination with an anti-CD20 monoclonal antibody (rituximab or ofatumumab). Thirty-eight (88%) patients received lenalidomide as frontline therapy and 5 (22%) as salvage therapy. In 2 patients lenalidomide was discontinued 2 and 3 months after treatment initiation because of grade > 3 infectious complications, and subsequent treatment started before response assessment; these two patients were included in the survival analysis, but not in the response assessment. In the 41 evaluable patients, the overall response rate (ORR) was 71%; complete remission (CR) rate was 24% with undetectable minimal residual disease in 2 of those patients (5%), and partial response (PR) rate was 46%. Median treatment duration was 11 months (range, 1-39 months) and 8 patients had < 2 months of treatment; at time of treatment discontinuation 28 patients (65%) were receiving a daily dose of ≥ 5 mg or higher whereas 15 (35%) were receiving a lower dose. The most common causes of treatment discontinuation were recurrent infections, peripheral neuropathy, persistent neutropenia, or fatigue (see Table for details).
Among all 43 patients, the median TTNT was 40 months (range, 1-96 months). Subsequent lines of therapy included BTK or PI3K inhibitors (13 patients), ofatumumab, obinutuzumab or rituximab with methylprednisolone (7 patients), chemoimmunotherapy (3 patients), or venetoclax (1 patient). The vast majority of patients responded to subsequent lines of therapy with an ORR of 95% and a CR rate of 45%. A univariate analysis showed that a long TTNT was associated with a clinical response (CR or PR) at time of treatment discontinuation (P= 0.01) and/or ≥ 2 months treatment duration (P=0.007) (Figure). The median overall survival of the 43 evaluated patients has not been reached at a median follow-up of 40 months (10-108 months) and 9 patients (21%) had died.
Conclusion. CLL patients whose lenalidomide treatment was discontinued due to toxicity experienced a long TTNT with a median duration of more than 40 months. These patients also showed a high response rate to subsequent lines of therapy with responses seen in 95% of patients. These findings suggest that the immunomodulatory effects of lenalidomide persist after treatment discontinuation. Correlative laboratory studies to determine whether lenalidomide induces a checkpoint inhibitor-like effect are ongoing.
Wierda: Janssen: Research Funding; Genentech/Roche: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Karyopharm: Research Funding; Emergent: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; GSK/Novartis: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Genzyme: Consultancy, Honoraria; The University of Texas MD Anderson Cancer Center: Employment; Kite: Research Funding; Juno: Research Funding; Acerta: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Merck: Consultancy, Honoraria. Jain: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Abbvie: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; Verastem: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding. Thompson: Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees. O'Brien: Acerta: Other: Research Support: Honorarium, Research Funding; Pharmacyclics: Consultancy, Other: Research Support: Honorarium, Research Funding; TG Therapeutics: Consultancy, Other: Research Support: Honorarium, Research Funding; Janssen: Consultancy; Aptose Biosciences, Inc.: Consultancy; Sunesis: Consultancy; ProNAI: Other: Research Support: Honorarium, Research Funding; Amgen: Consultancy; AbbVie: Consultancy; Pfizer: Consultancy, Research Funding; Alexion: Consultancy; Gilead Sciences, Inc.: Consultancy, Other: Research Support: Honorarium, Research Funding; Celgene: Consultancy; GSK: Consultancy; Astellas: Consultancy; Regeneron: Other: Research Support: Honorarium, Research Funding; CLL Global Research Foundation: Membership on an entity's Board of Directors or advisory committees; Vaniam Group LLC: Consultancy. Kantarjian: Delta-Fly Pharma: Research Funding; ARIAD: Research Funding; Novartis: Research Funding; Bristol-Meyers Squibb: Research Funding; Pfizer: Research Funding; Amgen: Research Funding. Burger: Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Novartis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal